ACT's First FDA SBIR Contract- Plasma Sterilization System
Advanced Cooling Technologies, Inc. (ACT) is pleased to announce that the Food and Drug Administration (FDA) will fund ACT’s Phase I SBIR proposal, “Ozone Plasma Sterilization System with Plasma-In-Lumen Technology”. In this project, ACT and Rutgers University will develop an ozone plasma-based sterilization system with the potential to replace conventional Ethylene Oxide (EtO) sterilization.
EtO sterilization is the most common method of sterilization used in medical equipment facilities because it is universally effective on any medical equipment containing heat-sensitive polymers and/or having high aspect ratios, like long lumens. However, due to the high cost of EtO, high toxicity and the length of time it takes to perform the sterilization using this method, the FDA SBIR program is funding research to develop alternative sterilization solutions. ACT and Rutgers University have identified an ozone plasma sterilization system with plasma-in-lumen technology (Figure 1) to be one innovative alternative solution offering many improvements over EtO.
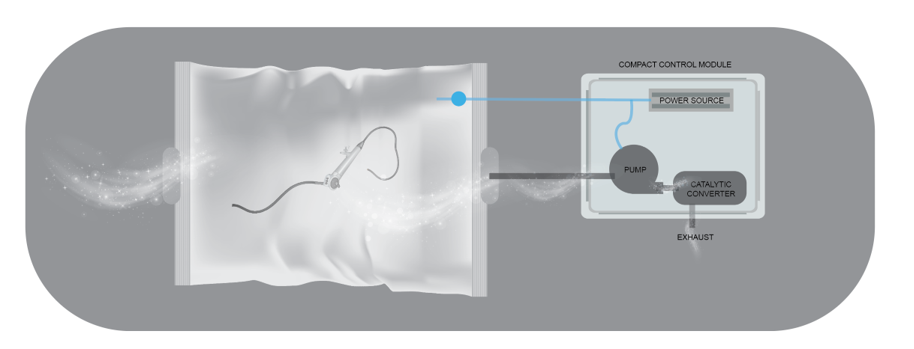
ACT and Rutgers University’s proposed system uses plasma to generate ozone (O3), a strong sterilizing agent. The plasma is generated inside a sterilization pouch that is made from a flexible sheet. The ozone plasma-based sterilization technique is also expected to sterilize medical scopes with long lumens. The system can also accommodate multiple pouches at once to increase sterilization throughput.
Dr. Yue Xiao will serve as the Principal Investigator and will work closely with the collaborators at RU: Prof. Aaron Mazzeo is a pioneer in flexible plasma reactors for decontamination and treatment, and Prof. Francois Berthiaume is an expert in metabolic and tissue engineering with extensive characterization experience.
“ACT’s research and development in non-thermal plasmas include chemical synthesis, methane reforming, and plasma surface treatment for improved aviation fuels,” said Dr. Rich Bonner, Vice President of R&D. “This new program will extend our non-thermal plasma research applications to include the medical field.”
Dr. Bill Anderson, Chief Engineer, added “ACT has more than $125 million in product sales from technologies seeded by SBIR and STTR programs. When completed, we hope to use this program to expand our offerings in the medical technology field.”
